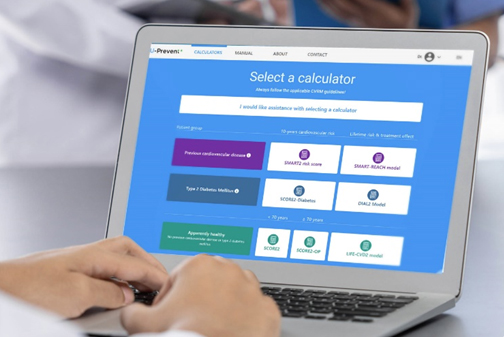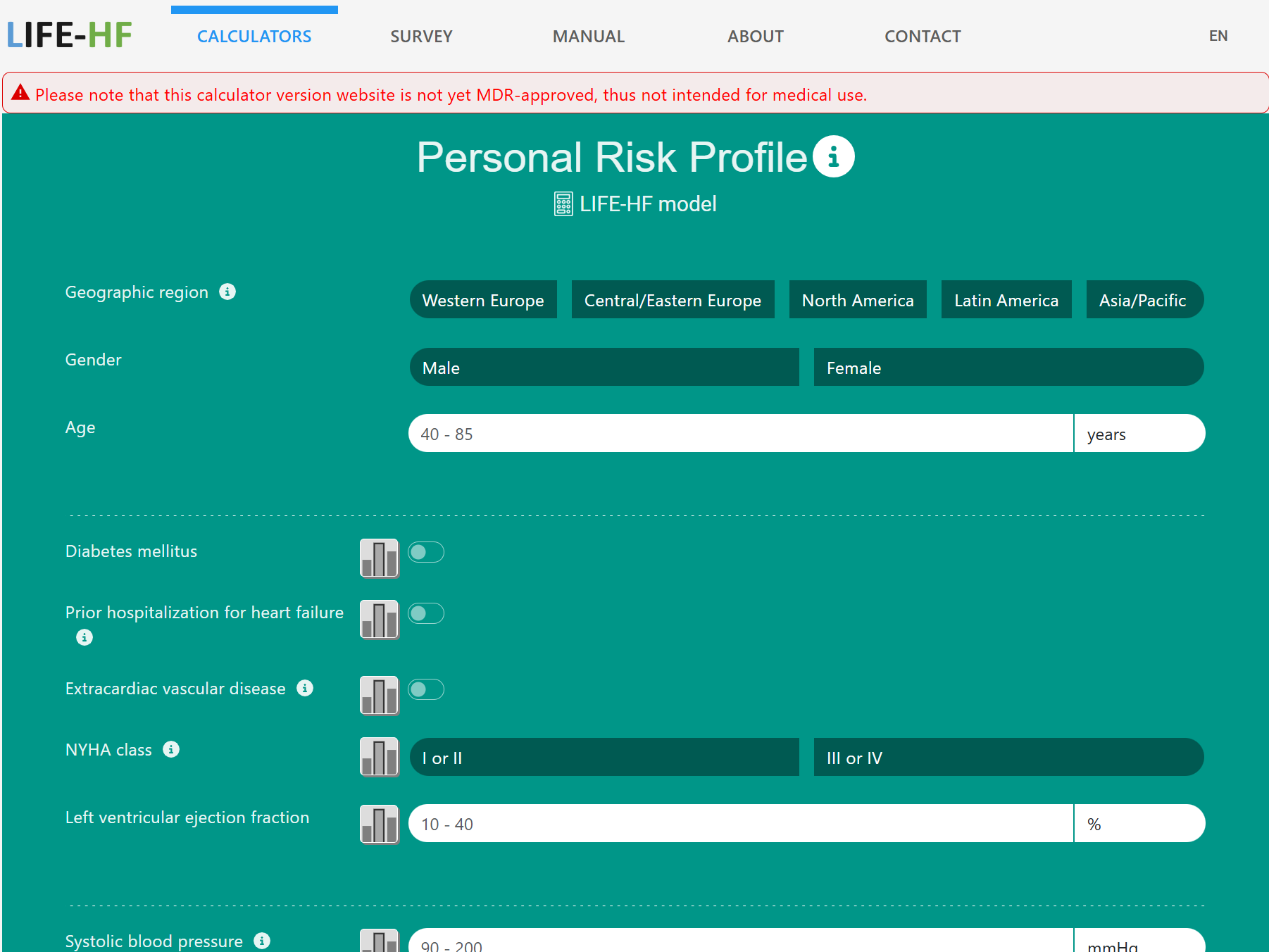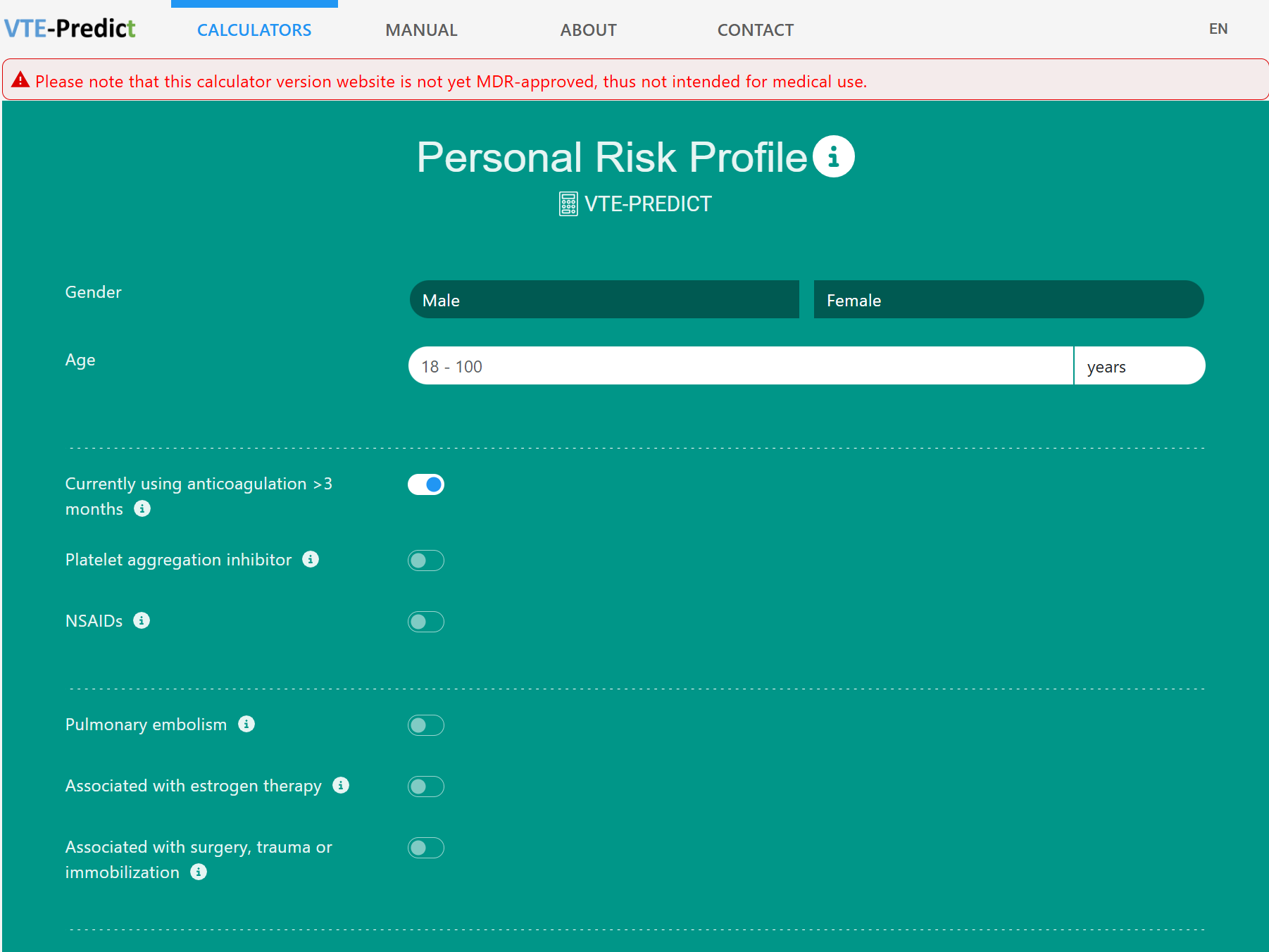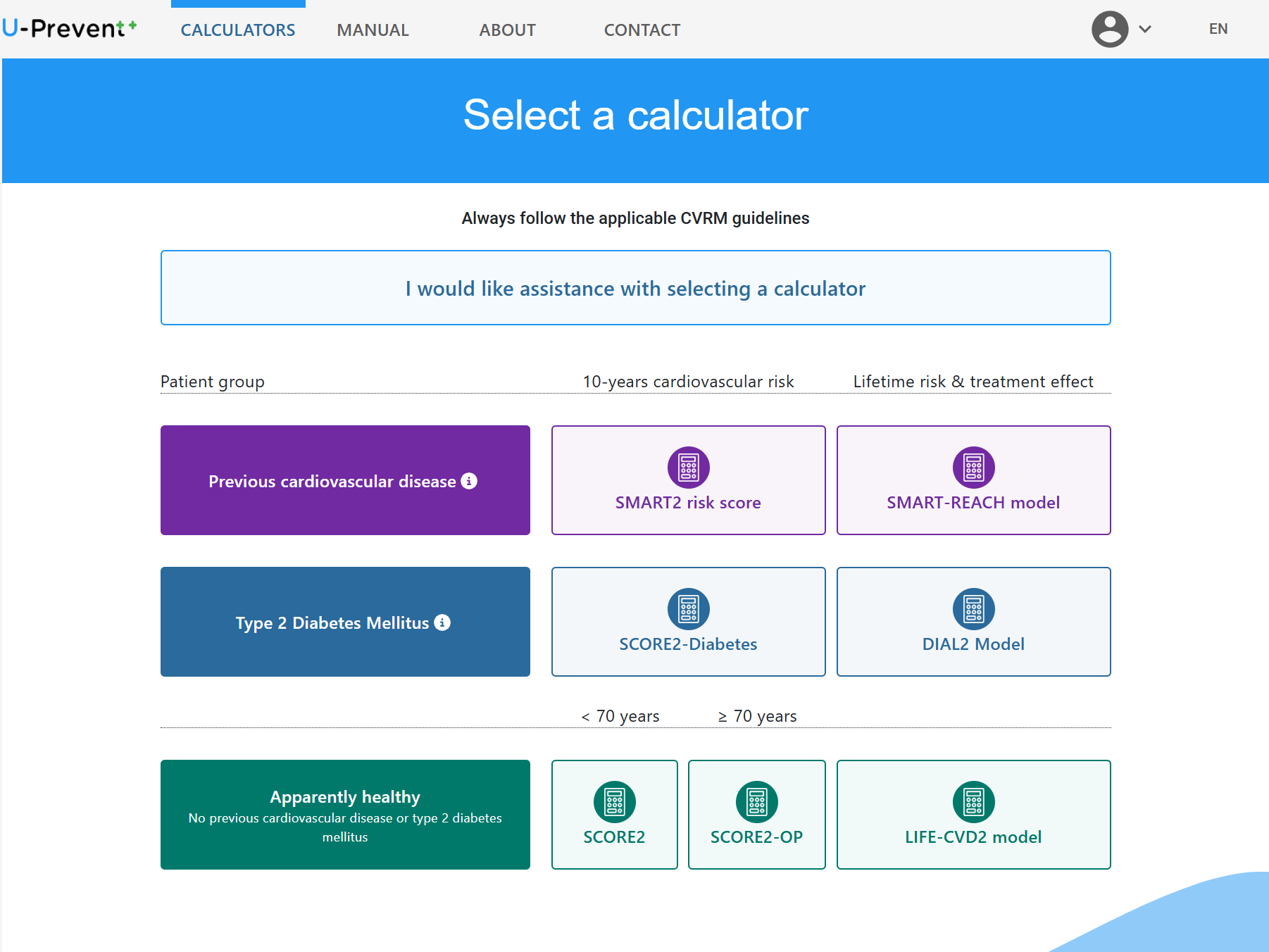
U-prevent is a clinical decision support system for the application of risk prediction models in clinical practice. The risk prediction calculators have been developed to determine an individual risk profile of developing cardiovascular disease and to analyze the effects of selected lifestyle andtreatment options. Supporting a shared decision-making process and providing patients with a better understanding of their risk and treatment options to lower that risk.
Join the more than 15.000 healthcare professionals with a registered U-Prevent account at www.u-prevent.com.