Ortec Logiqcare provides best practices for MDR-compliant CDSS production
In EU healthcare, prediction models deliver true clinical value only when converted into MDR-compliant Clinical Decision Support Systems (CDSS). CDSS development requires multidisciplinary collaboration—legal, regulatory, medical, data science, user experience, and software engineering. Ortec Logiqcare describes in a scientific paper preprint their best practices to produce CDSS (Figure 1).
CDSS should fit within the legal framework of the Medical Device Regulation (MDR) with its MDCG updates, the AI Act, the GDPR and NIS2, as well as at least 16 different IEC and/or ISO norms. These include safe and secure guidelines on information security, risk management, usability and manufacturing information. The prediction model should be assessed for data quality, fit for purpose, and scientific quality. The software development requires a dedicated production pipeline. Measures to guard quality for use should reduce user and data errors. Software implementation requires a clinical evaluation of the product, beyond its prediction model. After production, continued surveillance of the product and its usage is warranted.
Software development, implementation, and post-market surveillance require extensive documentation and traceability by an interdisciplinary team of medical, data, software, usability, and legal experts. This integrated approach ensures CDSS safety, effectiveness, reliability, regulatory compliance, and sustained clinical effectiveness in dynamic healthcare environments. These best practices facilitate to produce CDSS which minimizes patients harm and has proven clinical impact.
Read more about this publication in the preprint or contact John (dot) Jacobs (at) ortec (dot) com.
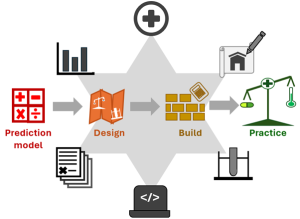
Figure 1. Best practices to produce CDSS.
Central to producing a CDSS is a validated prediction model is used to design user stories with appropriate test cases. This will be used to create a CDSS in the test environment, which after rigorous testing will be pushed to production (horizontal line in colours ranging red to green). This process is guarded by experts in data science, medical science, software design, software testing, writing software codes and the relevant laws and norms. These are indicated by black images, respectively, starting above the prediction model and following clockwise.
Overview of our Clinical Decision Support Solutions

